"A sea setting us on the ice has brought us close to danger." - Henry Hudson, 1607
In 1979 I was peering through the water at the bottom of the Hudson Strait. In advance of possible drilling for hydrocarbons southeast of Baffin Island we were looking at what was there. And we were amazed. As a marine biologist investigating benthos (the animals that live on the bottom of the ocean) my task was to identify the creatures and draw conclusions about the ecology of the area. To my astonishment the sea bottom teemed with life. Spidery crinoid lilies, stalked tunicates, super-flexible yet highly brittle serpent stars, sponges of a myriad forms, sea anemones waving scores of tentacles through the frigid water, scallops ready to jet propel themselves across the ocean bottom, branching bryozoans waving in the gentle currents, and many other animals difficult to identify in our photos of the sea bottom. Rather than the frigid wasteland that I had imagined, it was instead a submarine arctic jungle.
That was 33 years ago. Imagine my consternation to learn that in another 33 years large areas of the Arctic and sub-Antarctic oceans may become so corrosive that many of these animals may simply dissolve into oblivion.
Acid test: Ocean acidification
Since the beginning of the industrial revolution some 250 years ago, human civilization has been burning fossil fuels at an accelerating rate. The carbon dioxide emitted is a powerful greenhouse gas (GHG). The focus of much concern by environmentalists has been on the role of carbon dioxide in causing climate change. However, all the carbon dioxide that is emitted initially goes into the atmosphere, and since 71 per cent of the earth's surface is covered by oceans, much of that -- currently some 6 million tons of carbon dioxide per day -- dissolves into the water, creating carbonic acid. The oceans have absorbed roughly 30 per cent of the carbon dioxide caused by the burning of fossil fuels, and they contain vast reservoirs of dissolved carbon dioxide. Although as a percentage, the increase has been very small, about 3 per cent in surface waters and 0.25 per cent in the ocean overall, the effect has been quite large. Ocean acidity has increased by 30 per cent since pre-industrial levels (from pH 8.25 to 8.14) and modelling shows that if we continue to emit carbon dioxide at the current rate, ocean acidity will double (roughly to pH 7.9) by the end of the 21st century. This would be the largest change in the acidity of oceans to occur in many tens of millions of years.

Myriad animals and plants in the ocean -- mollusks (mussels, scallops, oysters, clams, snails), barnacles, sea urchins, starfish, crinoids, corals, coralline algae, foraminifera, and coccolithophores -- build their exoskeletons from calcium carbonate. Acidity dissolves calcium carbonate and all these creatures either have to work much harder in order to maintain their exoskeletons or else try and exist with thinner shells. Changing acidity affects the metabolic pathways of living organisms in ways that are problematic. Indeed, increasing acidity has had a negative effect on every marine animal that has been tested.
This is a global problem. Cold waters can absorb more carbon dioxide than warm ones so they become more acidic more rapidly. Forecasts are that by the middle of the 21st century large areas of the Arctic and sub-Antarctic oceans will be so corrosive that sea shells will begin to dissolve. Conversely, other problems will affect faunas of tropical waters where corals are abundant. Not only is the biodiversity of tropical coral reefs very high -- some 25 per cent of all marine species on the planet are found on them -- but the coral itself creates the physical substrate that provides the environment in which all these animals live. Corals are very vulnerable to pollution, changing water temperatures, and increased ocean acidity.
Dissolution in progress
A few illustrations of what is happening:
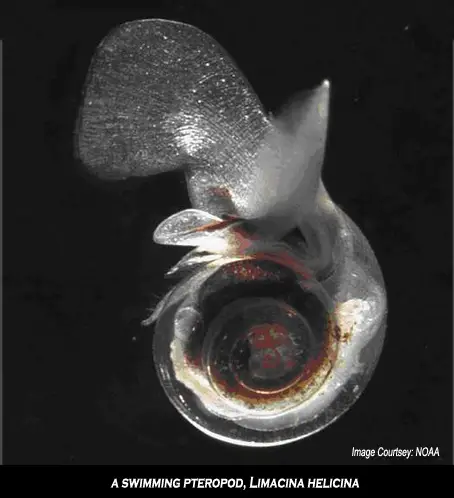
• Pteropods, colloquially known as sea butterflies for the way they swim, are pelagic snails. They are a critical food source for many animals in the Antarctic food web, sometimes present in thousands of individuals per cubic meter of seawater -- 20 per cent of all zooplankton. Pteropods have wafer thin shells composed of aragonite, a highly soluble form of calcium carbonate. Recent studies show that even a two-day exposure to the acidic conditions expected to occur by the middle of this century was sufficient to damage pteropod shells. Because of their ecological importance there are fears that if pteropod populations were to collapse it could cause a chain reaction extending from krill to whales.

• Coccolithophores, a kind of microscopic phytoplankton, respond variably to an increased acidity. One species, Emiliana huxleyi, has thickened its shell by 40 per cent over the last 230 years, but other species are unable to do so and become progressively decalcified. Coccolithophores are amongst the most important primary producers at the base of the marine food chain. They are also important in the global carbon cycle, absorbing carbon dioxide through photosynthesis and releasing it while producing their calcium carbonate plates. They also play a role in cloud formation, and hence temperature regulation of the climate. Coccolithophores release dimethyl sulphonioproprionate (DMSP) into the atmosphere. When converted into dimethyl sulphide (DMS) it promotes cloud condensation. Clouds, in turn, help to reflect solar radiation back into space and help cool the planet.
 • Foraminifera are amongst the commonest of marine plankton. They are
microscopic protozoans that collect food using a dynamic net of fine
strands of cytoplasm. As ocean waters acidify, the abundance and
diversity of foraminifera, as well as their ability to create their
calcium shells, all decline. When the pH of water is reduced (i.e., the
acidity is increased) to 7.8 or lower (roughly what forecasts say it
will be by the end of the 21st century) foraminifera are unable to form
their shells.
• Foraminifera are amongst the commonest of marine plankton. They are
microscopic protozoans that collect food using a dynamic net of fine
strands of cytoplasm. As ocean waters acidify, the abundance and
diversity of foraminifera, as well as their ability to create their
calcium shells, all decline. When the pH of water is reduced (i.e., the
acidity is increased) to 7.8 or lower (roughly what forecasts say it
will be by the end of the 21st century) foraminifera are unable to form
their shells.

• The northern abalone is a prized gourmet delicacy found from Baja California in Mexico north through British Columbia to Alaska. Over-fishing and poaching have already had a negative impact on this mollusk and have significantly reduced its populations in many areas. Recent experiments found that increasing the acidity of water killed 40 per cent of larvae, stunted the growth of those that survived, and increased the rate of shell abnormalities.
• The deleterious impacts of ocean acidification are not confined to animals and plants with calcium carbonate skeletons. They will ripple -- or perhaps tsunami -- through the ecosystems they are part of. Those shock waves may affect not only climate change but they will also impact human populations, particularly in the developing world.

Research conducted by Sarah Cooley at the Woods Hole Oceanographic Institute (WHOI) focused on countries such as Senegal, Madagascar, Gambia, Mozambique, and Haiti where shellfish are an important food item in many communities and where many people are already struggling with protein deficiencies in their diet. And not only are shellfish fisheries apt to suffer directly, but finfish fisheries as well since many species such as "haddock, halibut, herring, flounder, and cod, depend heavily on mollusks for their own nourishment." Beyond the dietary impacts are further economic ones. For example, some 30 per cent of the GDP of Tobago comes from direct and indirect coral reef tourism. If the coral reefs die imagine the effect on the society of the island.
Shell game: Canadian government inaction
What is being done to address this looming tragedy? In Canada, rather little. Rather than responding to these ringing alarm bells and racing to find solutions, the current federal government has immersed most of our environmental protections into an acid bath and is trying as hard as possible to see them dissolve. In the Harper Government's omnibus budget bill (C-38), currently before the Senate:
- The Environmental Assessment Act is being scrapped and replaced with a new and more limited one (which, tellingly, never mentions the words "climate change");
- Many provisions of the Environmental Assessment Agency are being undercut;
- The Kyoto Protocol Implementation Act is being killed;
- The Fisheries Act, the legislative bedrock of protection for fish, fisheries, and fishers is being gutted;
- The Navigable Waters Protection Act is being weakened to exempt pipelines and power lines;
- Decisions of the National Energy Board can now be reversed by cabinet, which makes the process of hearings, such as those being conducted on the Northern Gateway Pipeline, border on a farce;
- The Species at Risk Act is being compromised to allow various exemptions;
- The National Round Table on the Environment and Economy is being liquidated;
- Oil, gas, and nuclear regulations are being made less environmentally rigorous and more industry friendly;
- Environment Canada's Environmental Effects Monitoring Program will be scaled back.
Deep cuts to Environment Canada have curtailed a wide variety of environmental monitoring and regulatory programs. The government has cut funding to the Polar Environment Atmospheric Research Laboratory (PEARL), perhaps the single-most important research facility in the world in monitoring climate and environmental change in the high arctic. The Conservative Government is closing the Experimental Lakes Area (ELA), a globally significant research facility dedicated to ecosystem-scale research of aquatic habitats.
Not all of these measures relate directly to carbon emissions and ocean acidification, but some do, and collectively they indicate the degree to which the Harper Government regards environmental regulation as an impediment to untrammelled commercial development.
On an international front, in 2009 at the United Nations Climate Change conference in Copenhagen, Denmark the Canadian federal government did everything possible to obstruct constructive solutions (i.e., setting binding and necessary greenhouse gas reduction targets) earning Canada more "fossil awards" than any other country and a reputation for inaction among environmental organizations around the world. The following year in Cancún, Mexico, Canada continued to earn fossil awards as well as solidifying its reputation for obstruction and firmly leading from the rear. In 2011, in Durban, South Africa, Canada extended its winning streak (four consecutive fossil of the year awards, and two on the first day of the conference alone!) by its refusal to recommit to the Kyoto Protocol targets for greenhouse gas reductions -- the only country in the world to have signed the protocol and then torn it up. In Rio de Janeiro, Brazil where the Rio +20 Sustainable Development conference is only just beginning, Canada has already earned a reputation as the "Deleter of key principles and commitments."
From dissolution towards solution
The only way to stop ocean acidification -- and the only way to stop climate change -- is for all of us to emit less carbon dioxide. There's no way to gerrymander this. We can't postpone action on climate change and ocean acidification until economic conditions are ripe any more than we can postpone death or taxes. We must grapple with these issues now. Changing from a carbon consumptive and wasteful lifestyle in the developed world will entail adjustments. To characterize these as a decline in living standards is to mischaracterize them. Conspicuous, wasteful, pointless, consumption doesn't fundamentally enhance anyone's personal satisfaction, health, or well-being. However it undermines the quality of life -- and of environment -- for all in a pell-mell rush to the bottom of the tragedy of the commons. Turning our climate into a hothouse and our oceans into a corrosive brew would be a bleak monument to human stupidity, greed and shortsightedness. Now is the time to act before we create a corrosive oceanic wasteland.
Christopher Majka is a biologist, environmentalist, policy analyst, and arts advocate. He conducts research on the ecology, biodiversity and systematics of invertebrates, particularly beetles. He is a member of the Project Democracy team.
Original Article
Source: rabble.ca
Author: Christopher Majka
In 1979 I was peering through the water at the bottom of the Hudson Strait. In advance of possible drilling for hydrocarbons southeast of Baffin Island we were looking at what was there. And we were amazed. As a marine biologist investigating benthos (the animals that live on the bottom of the ocean) my task was to identify the creatures and draw conclusions about the ecology of the area. To my astonishment the sea bottom teemed with life. Spidery crinoid lilies, stalked tunicates, super-flexible yet highly brittle serpent stars, sponges of a myriad forms, sea anemones waving scores of tentacles through the frigid water, scallops ready to jet propel themselves across the ocean bottom, branching bryozoans waving in the gentle currents, and many other animals difficult to identify in our photos of the sea bottom. Rather than the frigid wasteland that I had imagined, it was instead a submarine arctic jungle.
That was 33 years ago. Imagine my consternation to learn that in another 33 years large areas of the Arctic and sub-Antarctic oceans may become so corrosive that many of these animals may simply dissolve into oblivion.
Acid test: Ocean acidification
Since the beginning of the industrial revolution some 250 years ago, human civilization has been burning fossil fuels at an accelerating rate. The carbon dioxide emitted is a powerful greenhouse gas (GHG). The focus of much concern by environmentalists has been on the role of carbon dioxide in causing climate change. However, all the carbon dioxide that is emitted initially goes into the atmosphere, and since 71 per cent of the earth's surface is covered by oceans, much of that -- currently some 6 million tons of carbon dioxide per day -- dissolves into the water, creating carbonic acid. The oceans have absorbed roughly 30 per cent of the carbon dioxide caused by the burning of fossil fuels, and they contain vast reservoirs of dissolved carbon dioxide. Although as a percentage, the increase has been very small, about 3 per cent in surface waters and 0.25 per cent in the ocean overall, the effect has been quite large. Ocean acidity has increased by 30 per cent since pre-industrial levels (from pH 8.25 to 8.14) and modelling shows that if we continue to emit carbon dioxide at the current rate, ocean acidity will double (roughly to pH 7.9) by the end of the 21st century. This would be the largest change in the acidity of oceans to occur in many tens of millions of years.

Myriad animals and plants in the ocean -- mollusks (mussels, scallops, oysters, clams, snails), barnacles, sea urchins, starfish, crinoids, corals, coralline algae, foraminifera, and coccolithophores -- build their exoskeletons from calcium carbonate. Acidity dissolves calcium carbonate and all these creatures either have to work much harder in order to maintain their exoskeletons or else try and exist with thinner shells. Changing acidity affects the metabolic pathways of living organisms in ways that are problematic. Indeed, increasing acidity has had a negative effect on every marine animal that has been tested.
This is a global problem. Cold waters can absorb more carbon dioxide than warm ones so they become more acidic more rapidly. Forecasts are that by the middle of the 21st century large areas of the Arctic and sub-Antarctic oceans will be so corrosive that sea shells will begin to dissolve. Conversely, other problems will affect faunas of tropical waters where corals are abundant. Not only is the biodiversity of tropical coral reefs very high -- some 25 per cent of all marine species on the planet are found on them -- but the coral itself creates the physical substrate that provides the environment in which all these animals live. Corals are very vulnerable to pollution, changing water temperatures, and increased ocean acidity.
Dissolution in progress
A few illustrations of what is happening:

• Pteropods, colloquially known as sea butterflies for the way they swim, are pelagic snails. They are a critical food source for many animals in the Antarctic food web, sometimes present in thousands of individuals per cubic meter of seawater -- 20 per cent of all zooplankton. Pteropods have wafer thin shells composed of aragonite, a highly soluble form of calcium carbonate. Recent studies show that even a two-day exposure to the acidic conditions expected to occur by the middle of this century was sufficient to damage pteropod shells. Because of their ecological importance there are fears that if pteropod populations were to collapse it could cause a chain reaction extending from krill to whales.

• Coccolithophores, a kind of microscopic phytoplankton, respond variably to an increased acidity. One species, Emiliana huxleyi, has thickened its shell by 40 per cent over the last 230 years, but other species are unable to do so and become progressively decalcified. Coccolithophores are amongst the most important primary producers at the base of the marine food chain. They are also important in the global carbon cycle, absorbing carbon dioxide through photosynthesis and releasing it while producing their calcium carbonate plates. They also play a role in cloud formation, and hence temperature regulation of the climate. Coccolithophores release dimethyl sulphonioproprionate (DMSP) into the atmosphere. When converted into dimethyl sulphide (DMS) it promotes cloud condensation. Clouds, in turn, help to reflect solar radiation back into space and help cool the planet.
 • Foraminifera are amongst the commonest of marine plankton. They are
microscopic protozoans that collect food using a dynamic net of fine
strands of cytoplasm. As ocean waters acidify, the abundance and
diversity of foraminifera, as well as their ability to create their
calcium shells, all decline. When the pH of water is reduced (i.e., the
acidity is increased) to 7.8 or lower (roughly what forecasts say it
will be by the end of the 21st century) foraminifera are unable to form
their shells.
• Foraminifera are amongst the commonest of marine plankton. They are
microscopic protozoans that collect food using a dynamic net of fine
strands of cytoplasm. As ocean waters acidify, the abundance and
diversity of foraminifera, as well as their ability to create their
calcium shells, all decline. When the pH of water is reduced (i.e., the
acidity is increased) to 7.8 or lower (roughly what forecasts say it
will be by the end of the 21st century) foraminifera are unable to form
their shells.
• The northern abalone is a prized gourmet delicacy found from Baja California in Mexico north through British Columbia to Alaska. Over-fishing and poaching have already had a negative impact on this mollusk and have significantly reduced its populations in many areas. Recent experiments found that increasing the acidity of water killed 40 per cent of larvae, stunted the growth of those that survived, and increased the rate of shell abnormalities.
• The deleterious impacts of ocean acidification are not confined to animals and plants with calcium carbonate skeletons. They will ripple -- or perhaps tsunami -- through the ecosystems they are part of. Those shock waves may affect not only climate change but they will also impact human populations, particularly in the developing world.

Research conducted by Sarah Cooley at the Woods Hole Oceanographic Institute (WHOI) focused on countries such as Senegal, Madagascar, Gambia, Mozambique, and Haiti where shellfish are an important food item in many communities and where many people are already struggling with protein deficiencies in their diet. And not only are shellfish fisheries apt to suffer directly, but finfish fisheries as well since many species such as "haddock, halibut, herring, flounder, and cod, depend heavily on mollusks for their own nourishment." Beyond the dietary impacts are further economic ones. For example, some 30 per cent of the GDP of Tobago comes from direct and indirect coral reef tourism. If the coral reefs die imagine the effect on the society of the island.
Shell game: Canadian government inaction
What is being done to address this looming tragedy? In Canada, rather little. Rather than responding to these ringing alarm bells and racing to find solutions, the current federal government has immersed most of our environmental protections into an acid bath and is trying as hard as possible to see them dissolve. In the Harper Government's omnibus budget bill (C-38), currently before the Senate:
- The Environmental Assessment Act is being scrapped and replaced with a new and more limited one (which, tellingly, never mentions the words "climate change");
- Many provisions of the Environmental Assessment Agency are being undercut;
- The Kyoto Protocol Implementation Act is being killed;
- The Fisheries Act, the legislative bedrock of protection for fish, fisheries, and fishers is being gutted;
- The Navigable Waters Protection Act is being weakened to exempt pipelines and power lines;
- Decisions of the National Energy Board can now be reversed by cabinet, which makes the process of hearings, such as those being conducted on the Northern Gateway Pipeline, border on a farce;
- The Species at Risk Act is being compromised to allow various exemptions;
- The National Round Table on the Environment and Economy is being liquidated;
- Oil, gas, and nuclear regulations are being made less environmentally rigorous and more industry friendly;
- Environment Canada's Environmental Effects Monitoring Program will be scaled back.
Deep cuts to Environment Canada have curtailed a wide variety of environmental monitoring and regulatory programs. The government has cut funding to the Polar Environment Atmospheric Research Laboratory (PEARL), perhaps the single-most important research facility in the world in monitoring climate and environmental change in the high arctic. The Conservative Government is closing the Experimental Lakes Area (ELA), a globally significant research facility dedicated to ecosystem-scale research of aquatic habitats.
Not all of these measures relate directly to carbon emissions and ocean acidification, but some do, and collectively they indicate the degree to which the Harper Government regards environmental regulation as an impediment to untrammelled commercial development.
On an international front, in 2009 at the United Nations Climate Change conference in Copenhagen, Denmark the Canadian federal government did everything possible to obstruct constructive solutions (i.e., setting binding and necessary greenhouse gas reduction targets) earning Canada more "fossil awards" than any other country and a reputation for inaction among environmental organizations around the world. The following year in Cancún, Mexico, Canada continued to earn fossil awards as well as solidifying its reputation for obstruction and firmly leading from the rear. In 2011, in Durban, South Africa, Canada extended its winning streak (four consecutive fossil of the year awards, and two on the first day of the conference alone!) by its refusal to recommit to the Kyoto Protocol targets for greenhouse gas reductions -- the only country in the world to have signed the protocol and then torn it up. In Rio de Janeiro, Brazil where the Rio +20 Sustainable Development conference is only just beginning, Canada has already earned a reputation as the "Deleter of key principles and commitments."
From dissolution towards solution
The only way to stop ocean acidification -- and the only way to stop climate change -- is for all of us to emit less carbon dioxide. There's no way to gerrymander this. We can't postpone action on climate change and ocean acidification until economic conditions are ripe any more than we can postpone death or taxes. We must grapple with these issues now. Changing from a carbon consumptive and wasteful lifestyle in the developed world will entail adjustments. To characterize these as a decline in living standards is to mischaracterize them. Conspicuous, wasteful, pointless, consumption doesn't fundamentally enhance anyone's personal satisfaction, health, or well-being. However it undermines the quality of life -- and of environment -- for all in a pell-mell rush to the bottom of the tragedy of the commons. Turning our climate into a hothouse and our oceans into a corrosive brew would be a bleak monument to human stupidity, greed and shortsightedness. Now is the time to act before we create a corrosive oceanic wasteland.
Christopher Majka is a biologist, environmentalist, policy analyst, and arts advocate. He conducts research on the ecology, biodiversity and systematics of invertebrates, particularly beetles. He is a member of the Project Democracy team.
Original Article
Source: rabble.ca
Author: Christopher Majka

No comments:
Post a Comment